News
Latest information

Summary
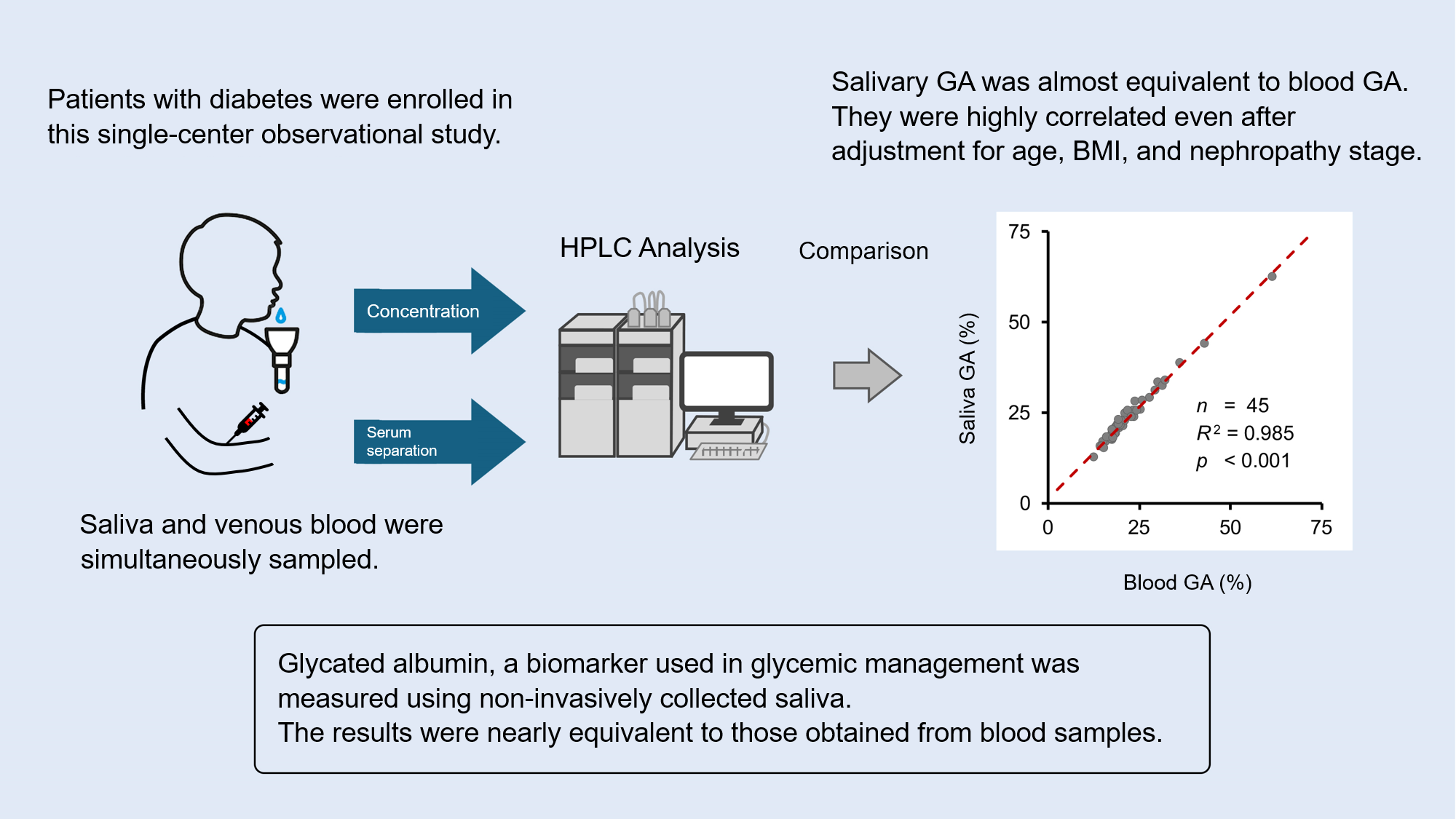
A research group led by Assistant Professor Masakazu Aihara of the Department of Diabetes and Metabolic Diseases, University of Tokyo Hospital, Professor Naoto Kubota of the Department of Metabolic Medicine, Faculty of Life Science, Kumamoto University, and Koshin Sekimizu, CEO of Provigate Inc., a medical-engineering start-up company from the University of Tokyo, has reported that a newly developed assay method for salivary glycated albumin levels gave almost equivalent results to the results from a conventional blood test.
Hemoglobin A1c (HbA1c) levels are commonly used marker for glycemic management in diabetes. The GA level is also used to indicate short-term blood glucose management. However, since these tests require blood sampling, the burden and invasiveness of blood sampling for frequent testing should be considered. Self-monitoring of blood glucose (SMBG) with a portable device or continuous glucose monitoring (CGM) with a wearable device has also been used, but the invasiveness of blood sampling by frequent finger-pricking for SMBG or of the fiber implantation with needle piercing for CGM has been a barrier to expand their use.
The outcome of this project could lead to the realization of a completely noninvasive post-in salivary test. By combining it with a smartphone application that could directly return the result from the testing laboratory to the subject, implementation of an easier and more frequent home blood glucose management method could be expected.
Details
【Background of the study】
Glycemic management through diet and physical activity is important for diabetes care, in addition to medication. Glucose monitoring at an appropriate frequency is inevitable to monitor the effects of these activities and lifestyle management. For this purpose, home testing devices such as self-monitoring of blood glucose (SMBG) device that measures blood glucose levels at any moment in self-sampled fingerprick blood or a continuous monitoring (CGM) device that continuously measures glucose levels in interstitial fluid with a sensor implanted under the skin were used, in addition to hemoglobin A1c (HbA1c), blood glucose, and glycated albumin (GA) tests those are measured by blood sampling at hospital visits.
Even though these biomarkers and their measurement methods have been established for screening diabetes and monitoring glucose levels, there are still issues that cannot be resolved by either method to monitor the outcomes of lifestyle management at home. HbA1c levels are useful markers for diagnosis and long-term glycemic control, but they change slowly and are not suitable to grab rapid changes. On the other hand, SMBG requires frequent blood samplings and measurements, in addition to expertise, to obtain a whole picture of glucose levels that change on diet, physical activity, and sleep, since the glucose level measurements only provide an instantaneous blood glucose level. CGM devices can continuously monitor blood glucose fluctuations over a period of 10 to 14 days. However, it is also associated with the cost and burden of puncture with an applicator, subcutaneous filament placement, and living with the device attached to the skin. Therefore, many people with diabetes are actually forced to rely only on HbA1c levels, which are measured once a few months of hospital visits, and continue nutrition and exercise therapy.
As a potential solution, the development of smartwatch-type noninvasive blood glucose meters is often reported in the mass media. However, no wearable non-invasive blood glucose meter that achieves practical accuracy has been commercialized to date. The U.S. Food and Drug Administration (FDA) and the Japan Diabetes Society have issued warnings about the health risks associated with the use of unapproved devices claiming such capabilities (Ref. 1). To overcome this situation, a research group led by Assistant Professor Masakazu Aihara of the Department of Diabetes and Metabolic Diseases, University of Tokyo Hospital, Professor Naoto Kubota of the Department of Metabolic Medicine, Faculty of Life Science, Kumamoto University, and Koshin Sekimizu, CEO of Provigate Inc., has been searching for a practical and non-invasive method of blood glucose management through a completely different approach. The group has reported that albumin can be collected from saliva and tear fluid, those can be collected noninvasively, and that GA levels can be analyzed using them (Ref. 2), a method for analyzing GA levels from a drop of blood collected by finger-pricking (Ref. 3), and that once-weekly GA measurement at home, in addition to standard medical treatment, improved blood glucose levels in people with type 2 diabetes (Ref. 4). This study further expanded these studies and newly developed a more practical clinical test method using saliva samples. The performance of this method was evaluated by comparing it to that of conventional blood tests with the cooperation of people hospitalized for blood glucose management in diabetes.
【Method】
This observational study was performed in a single institute. With the cooperation of people hospitalized for glycemic management of diabetes, both blood and saliva samples were collected during fasting and postprandial periods within three days of admission and during fasting within three days before discharge. Glycated albumin levels of saliva samples measured with the newly developed high-performance liquid chromatography (HPLC) method were compared with those of blood samples analyzed with a conventional method. The effects of potentially confounding factors were also analyzed.
【Results】
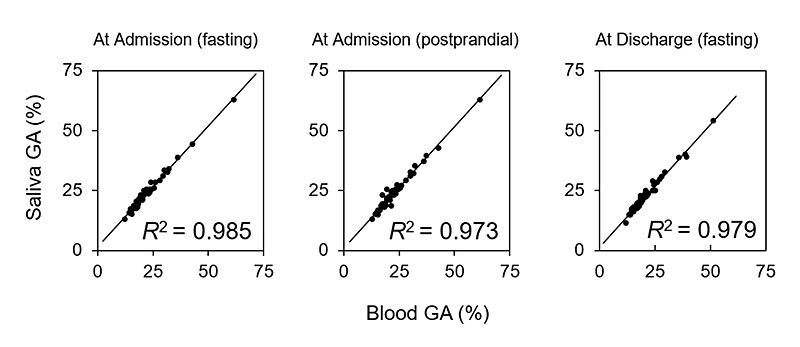
A total of 144 samples were analyzed from 56 individuals with type 1 and type 2 diabetes (168 samples in total), of which 2 samples had insufficient blood volume, 11 samples had insufficient saliva volume, 4 samples were defective in sample preparation, and 7 samples were found to have insufficient sample concentration after analysis. The coefficient of determination was very high for the samples collected at admission (n = 45, R2 = 0.985 for the sampling at fasting and n=48, R2=0.973 for postprandial sampling), and those collected at discharge (n=51, R2=0.979, at fasting). Multivariate analysis adjusted for age, BMI, and diabetic nephropathy stage as confounders also showed similarly significant correlations.
【Discussion and Future Prospect】
This study revealed that GA levels can be measured from noninvasively sampled saliva samples accurately as well as conventional bood samples. Combining with previously developed technology for post-in finger-prick blood tests, this method could be expanded to once-weekly home salivary glycated albumin monitoring. Such techniques could complement conventional testing methods to provide a completely non-invasive method for glycemic management for diabetes.
Researcher Information
Masakazu Aihara, MD., Ph.D. (Assistant Professor, Department of Diabetes and Metabolic Diseases, University of Tokyo Hospital)
Toshimasa Yamauchi, MD., Ph.D. (Professor, Department of Diabetes and Metabolic Diseases, University of Tokyo Hospital)
Naoto Kubota, MD., Ph.D. (Professor, Department of Metabolic Medicine, Faculty of Life Science, Kumamoto University)
Koshin Sekimizu, Ph.D. (CEO, Provigate Inc.)
Publication Information
Journal:Diabetes Research and Clinical Practice
Title:Salivary glycated albumin could be as reliable a marker of glycemic control as blood glycated albumin in people with diabetes.
Authors:Masakazu Aihara, Kouji Yano, Tomoko Irie, Mitsumi Nishi, Kenji Yachiku, Itsushi Minoura, Koshin Sekimizu, Yoshitaka Sakurai, Takashi Kadowaki, Toshimasa Yamauchi, Naoto Kubota
DOI:10.1016/j.diabres.2024.111903
Publication date:Oct. 22, 2024
References
1)US Food and Drug Administration, Do Not Use Smartwatches or Smart Rings to Measure Blood Glucose Levels: FDA Safety Communication (2024/2/21)
https://www.fda.gov/medical-devices/safety-communications/do-not-use-smartwatches-or-smart-rings-measure-blood-glucose-levels-fda-safety-communication
2)Aihara M, Jinnouchi H, Yoshida A, et al. Evaluation of glycated albumin levels in tears and saliva as a marker in patients with diabetes mellitus. Diabetes Res Clin Pract. 2023; 199:110637,
https://doi.org/10.1016/j.diabres.2023.110637
3)Aihara M, Irie T, Yasukawa K, et al. Development of a high-performance liquid chromatographic glycated albumin assay using finger-prick blood samples. Clin Chim Acta. 2023; 542:117272.
https://doi.org/10.1016/j.cca.2023.117272
4)Hideaki Jinnouchi, Akira Yoshida, Mariko Taniguchi, et al., Efficacy of self-review of lifestyle behaviors with once-weekly glycated albumin measurement in people with type 2 diabetes: a randomized pilot study. Diabetes Therapy 2024; 1561–1575.
https://doi.org/10.1007/s13300-024-01599-2
Funding
This study was supported by a grant for young researchers from the Japan Association for Diabetes Education and Care (to M.A.), a grant from TERUMO Life Science Foundation (to M.A.), and a grant from the Japan Agency for Medical Research and Development (AMED).
Contact
Dr. Koshin Sekimizu
President and CEO, Provigate, Inc.
https://provigate.com/en/contact/
People may think of glucose monitoring as simple as this: "Diabetes is a disease of blood sugar. This is why people with diabetes use glucometer."
However, it's not that simple. Blood sugar measurement has diverse objectives, including diagnosing diabetes, dosing self-injection of insulin, avoiding hypoglycemia due to excessive drug efficacy, and behavior change.
You must choose the appropriate blood sugar measurement method for your purpose.
There are two types of self-blood glucose measurement methods: SMBG (Self-Monitoring of Blood Glucose), which is widely used, and CGM (Continuous Glucose Monitoring), which has become popular in recent years.
These two blood glucose measurement methods are designed mainly for patients who self-inject insulin and other injectable drugs.
Until now, there has been no simple and daily method for measuring blood glucose at home other than these two methods.
Some drugs, such as insulin, are very potent and can cause dangerous hypoglycemia if the dosage is incorrect.
Therefore, for example, those who use insulin must measure their blood glucose accurately before self-injection at home and carefully determine the dose.
After injection, if there are signs of hypoglycemia, it is necessary to measure blood glucose immediately. If necessary, you need to take some sugar to avoid hypoglycemia.
However, both methods have an issue with invasiveness. In addition, they cost a lot. CGM costs at least $60 and needs to be replaced every two weeks, which is also a significant economic burden. SMBG requires frequent measurements, so the total cost becomes significant when accumulated.
Unfortunately, SMBG and CGM are not suitable for everyone due to invasiveness and cost.
At Provigate, we are looking for individuals who genuinely care about each user, are passionate about supporting health, and want to work together to create a new future for healthcare and medicine.
If you’re someone who wants to leverage your experience as a healthcare professional, enjoys the fusion of technology and medicine, and is eager to take on new challenges, we’d love for you to join us!
Copyright© Provigate, Inc. All Rights Reserved.