News
Latest information

Summary
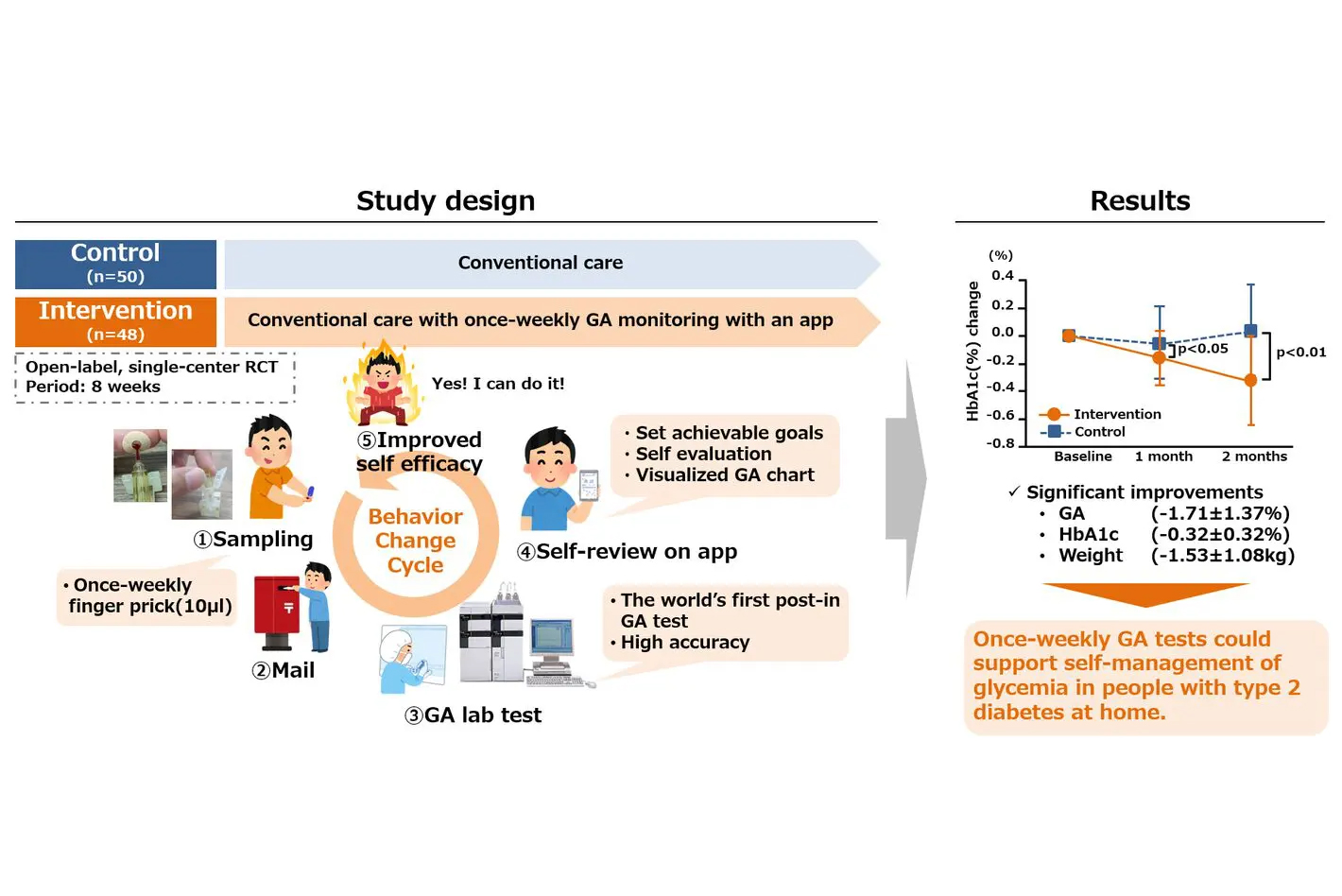
A joint research group of Jinnouchi Hospital, the University of Tokyo Hospital, the Kumamoto University Hospital and Provigate found that once-weekly home glycated albumin (GA) monitoring in combination with a behavior change app significantly improved blood glucose levels, weight and other parameters in people with type 2 diabetes.
GA levels are expected to change sensitively in response to changes in average blood glucose levels over the past week. Therefore, if GA levels are measured once a week, changes in lifestyle habits that affect blood glucose levels, such as diet, exercise, and medication, over the past week can be easily quantified as changes in GA levels. By using GA monitoring, users can easily review and modify their behavior to improve glucose control. However, no studies have been reported to date on measuring GA values at home and utilizing them for behavior change.
Following the results, the research team is also working on the research and development of easier and less invasive home rapid testing (POCT) methods and saliva postal testing methods, as well as improving apps. These are expected to lead to better diabetes treatment in the future.
The results were published online on 16 May (CET) in Diabetes Therapy.
Details
【Introduction】
Today, self-monitoring of blood glucose (SMBG), which measures instantaneous blood glucose by finger pricking, continuous glucose monitoring (CGM), which measures interstitial fluid glucose continuously by an implanted sensor, and HbA1c, which indirectly measures average blood glucose over one or two months are used in clinical practice for diabetes as indicators of glucose control. SMBG and CGM are essential for determining injection doses and avoiding hypoglycemia, especially for users of injectable anti-diabetic drugs such as insulin. HbA1c is also a reliable diagnostic criteria element for diabetes.
However, from the perspective of a tool for routine monitoring and behavior change in people with diabetes (PWD), neither of these methods is ideal: SMBG only provides blood glucose at the moment of measurement and requires frequent finger pricking per day to get a full picture of blood glucose variability; CGM requires the implantation of a subcutaneous filament-like sensor, which must be replaced by a puncture with an applicator every 10 to 14 days. All of these are costly and pose challenges in terms of invasiveness, cost and ease of use for all PWDs. In addition, while HbA1c is useful for diagnosing diabetes and as a target for long-term glycemic control, it is not suitable as a behavior change indicator because it changes slowly, making it difficult to reflect recent blood sugar changes. What is needed is an affordable, minimally or non-invasive and simple blood glucose measurement method.
Therefore, Dr Toshiya Sakata of the University of Tokyo’s Graduate School of Engineering and Dr Naoto Kubota of the University of Tokyo’s Graduate School of Medicine (at the time) and their colleagues have been working for over 10 years towards establishing innovative blood glucose monitoring methods. In the early years, the project was financially supported by the Japan Science and Technology Agency (JST) “START“ project[1] granted in 2012. In 2015, Provigate Inc. was founded as a startup based on the results of the project.
In 2016, the New Energy and Industrial Technology Development Organization (NEDO) adopted the “STS” project[2], in which we found a strong correlation between tear fluid and blood GA levels and the possibility of a completely non-invasive blood glucose monitoring method.
GA indicates the ratio of glycation of albumin molecules in the blood. It is known that albumin glycation rises and falls in proportion to blood glucose levels and has a very short half-life of 17 days. Therefore, if GA values are measured once a week, blood glucose fluctuations over the last week or so can be easily quantified and visualized as changes in GA% values. Even more usefully, GA values are measured as an intramolecular ratio of albumin rather than as a concentration in body fluids, so even tear fluid or saliva may be accurately measured. In addition to being a weekly test, the fact that it can also be used with tear fluid and saliva makes it a promising method of blood glucose monitoring that is affordable, minimally or non-invasive and easy to use.
In Japan and some other countries, the majority of non-insulin PWDs are not covered by public insurance to use SMBG or CGM. Thus, they can only measure their blood glucose when they visit the hospital once every 1-3 months. If GA testing could be done weekly at home and use the app to review their behavior over the past week, which could lead to lifestyle improvements.
The research team has therefore advanced the development of GA biosensors with the financial support of the AMED “Advanced Measurement Project” in 2018 [3], and has shown that GA of tear fluid and saliva strongly correlates with GA of blood (Ref. 1), and that the biweekly measurement of GA at a hospital can induce behavioral changes in PWDs and prevent blood glucose from worsening (Ref. 2). Furthermore, with another financial support of the NEDO “SCA” project in 2019 [4] and the NEDO ”PCA” project in 2020 [5], we were able to complete the development of elemental technologies for GA biosensors and medical device prototypes. In addition, the joint research has established the world’s first home testing method for GA, a stable post-in testing method (HPLC method) using finger-prick blood samples (Ref. 3).
Based on these research results, and with another financial support of the AMED “Health and Medical Information Project” in 2021 [6], a research group led by Dr Hideaki Jinnouchi, Director of Jinnouchi Hospital, Masakazu Aihara, Assistant Professor at the Department of Diabetes and Metabolism, Tokyo University Hospital, Naoto Kubota, Professor at the Department of Diabetes, Metabolism and Endocrinology (Graduate School of Life Sciences), Kumamoto University Hospital and Koshin Sekimizu, CEO of Provigate Inc. have investigated whether weekly home post-in GA monitoring in combination with an app improves blood glucose control in non-insulin people with type 2 diabetes who do not use blood glucose self-monitoring.
【Methods】
The study was conducted as a single-center, open-labelled, randomized controlled trial. The intervention period was 8 weeks and was conducted in Japan in non-insulin people with type 2 diabetes with HbA1c levels between 7.0% and 9.0%. In contrast to the control group, which received only conventional treatment, the intervention group received, in addition to conventional treatment, home post-in GA monitoring and daily brief self-review of lifestyle behavior using a smartphone app.
【Key results】
98 participants (72.0% male, age 63.2 ± 11.4, HbA1c 7.39 ± 0.39%) were randomly assigned to the intervention and control groups. In the intervention group, significant reductions in GA and HbA1c values were observed from baseline to the last observation date (-1.71 ± 1.37% and -0.32 ± 0.32%, respectively). Significant reductions in body weight, waist circumference and calorie consumption (p < 0.0001, p = 0.0003 and p = 0.0346, respectively) were also observed, but no significant reduction in calorie intake was observed (p = 0.678).
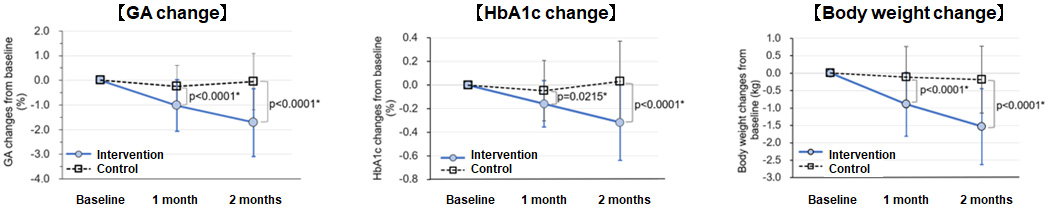
【Discussion】
This study represents the first report on GA monitoring at home and its application for behavior change. Once-weekly GA monitoring using an app shows promise as a low-frequency, affordable, and minimally invasive home blood glucose monitoring method. The research team has established the OMEGA Study Group[7], which aims to clinically apply GA. Additionally, we are working on developing a home rapid testing (POCT) method using finger-prick blood and a saliva-based GA post-in testing method while also enhancing the app. We anticipate that future GA testing research results will contribute to improved diabetes treatment.
Joint research team
Hideaki Jinnouchi, MD, PhD(Director, Jinnouchi Hospital)
Masakazu Aihara, MD, PhD(Assistant Professor, The Univesity of Tokyo Hospital)
Naoto Kubota, MD, PhD(Professor, Kumamoto University Hospital)
Koshin Sekimizu, PhD(President and CEO, Provigate, Inc.)
Journal information
Journal:Diabetes Therapy
Title:Efficacy of self-review of lifestyle behaviors with once-weekly glycated albumin measurement in people with type 2 diabetes: a randomized pilot study.
Authors:Hideaki Jinnouchi, MD, PhD; Akira Yoshida, PhD; Mariko Taniguchi; Eisaku Yamauchi; Daisuke Kurosawa; Kenji Yachiku, MD, MHA; Itsushi Minoura, PhD; Takashi Kadowaki, MD, PhD; Toshimasa Yamauchi, MD, PhD; Masakazu Aihara, MD, PhD; Naoto Kubota, MD, PhD; Koshin Sekimizu, PhD*
DOI:https://doi.org/10.1007/s13300-024-01599-2
References
1) Aihara M, Jinnouchi H, Yoshida A, et al. Evaluation of glycated albumin levels in tears and saliva as a marker in patients with diabetes mellitus. Diabetes Res Clin Pract. 2023;199:110637.
https://doi.org/10.1016/j.diabres.2023.110637
2) Masakazu Aihara, Takanori Hayashi, Chie Koizumi, et al. Bi-weekly glycated albumin measurement was useful to encourage behavioral changes in people with type 2 diabetes mellitus. Diabetes Therapy. 2023.
https://doi.org/10.1007/s13300-023-01452-y
3) Aihara M, Irie T, Yasukawa K, et al. Development of a high-performance liquid chromatographic glycated albumin assay using finger-prick blood samples. Clin Chim Acta. 2023;542:117272.
https://doi.org/10.1016/j.cca.2023.117272
Grant
This research was supported by the AMED “Health and Medical Information” granted in 2021.
Footnotes
[1] FY2012 JST START project “Research and development to achieve the practical use of a semiconductor-based biosensor for non-invasive IVDs – Efforts to reduce the burden of people with diabetes by utilizing blood sampling-free glucose sensors”.
[2] FY2016 NEDO STS project ‘Development of a tear fluid clinical testing platform’
[3] FY 2018 AMED Advanced Measurement Project ‘Research and development of a glucose monitoring device with a motivation-evoking glycemic control indicator’
[4] FY 2019 NEDO SCA project ‘Development of motivation-evoking systems to monitor blood glucose level at home’
[5] FY2020 NEDO PCA project ‘Development of an IoT home blood glucose monitoring system’
[6] FY2021 AMED Health and Medical Information Project “Development of a system for inducing behavioral change and preventing progression in people with diabetes by sharing weekly glycated albumin testing data between medical professionals and users”
[7] https://omega-study.org/
OMEGA Study Group
The OMEGA Study Group integrates minimally invasive and non-invasive blood glucose monitoring technologies, leveraging GA monitoring and communication tools like smartphone apps. Their mission is to develop and disseminate solutions that enhance the quality of life for individuals with diabetes or those at risk.
Website:https://omega-study.org/
Contact
【About Research】
Jinnouchi Hospital
Dr. Akira Yoshida
Director of Department of Pharmacy, Director of Clinical Trial Unit
The University of Tokyo Hospital
Dr. Masakazu Aihara
Assistant Professor, Department of Diabetes and Metabolism
*Please contact through the Public Relations Center at the University of Tokyo Hospital
e-mail:pr@adm.h.u-tokyo.ac.jp
Kumamoto University Hospital
Dr. Naoto Kubota
Professor, Department of Diabetes, Metabolism and Endocrinology (Graduate School of Life Sciences)
*Please contact through the Public Relations Strategy Office, General Affairs Division, General Affairs Department, National University Corporation Kumamoto University
e-mail:sos-koho@jimu.kumamoto-u.ac.jp
【About GA monitoring technology and the app】
Dr. Koshin Sekimizu
President and CEO, Provigate, Inc.
e-mail:info@provigate.com
【About OMEGA Study Group】
e-mail:admin@omega-study.org

People may think of glucose monitoring as simple as this: "Diabetes is a disease of blood sugar. This is why people with diabetes use glucometer."
However, it's not that simple. Blood sugar measurement has diverse objectives, including diagnosing diabetes, dosing self-injection of insulin, avoiding hypoglycemia due to excessive drug efficacy, and behavior change.
You must choose the appropriate blood sugar measurement method for your purpose.
There are two types of self-blood glucose measurement methods: SMBG (Self-Monitoring of Blood Glucose), which is widely used, and CGM (Continuous Glucose Monitoring), which has become popular in recent years.
These two blood glucose measurement methods are designed mainly for patients who self-inject insulin and other injectable drugs.
Until now, there has been no simple and daily method for measuring blood glucose at home other than these two methods.
Some drugs, such as insulin, are very potent and can cause dangerous hypoglycemia if the dosage is incorrect.
Therefore, for example, those who use insulin must measure their blood glucose accurately before self-injection at home and carefully determine the dose.
After injection, if there are signs of hypoglycemia, it is necessary to measure blood glucose immediately. If necessary, you need to take some sugar to avoid hypoglycemia.
However, both methods have an issue with invasiveness. In addition, they cost a lot. CGM costs at least $60 and needs to be replaced every two weeks, which is also a significant economic burden. SMBG requires frequent measurements, so the total cost becomes significant when accumulated.
Unfortunately, SMBG and CGM are not suitable for everyone due to invasiveness and cost.
Let’s make significant innovations in the era of the healthcare revolution.
We’re looking for talented and enthusiastic individuals to create a better future for everyone.
Copyright© Provigate, Inc. All Rights Reserved.